
/GettyImages-604346724-589a56263df78caebc80b0c5.jpg)
These elements were arranged in the periodic table as per their atomic weights. At the time of Mendeleev, only 63 elements were known. The periodic table organised the vast quantity of information accessible for each element. He noticed the periodicity when the elements were positioned according to their atomic weights. He developed the Periodic law based on his extensive research on the periodicity of physical and chemical properties of elements. Mendeleev, a Russian chemist, made the first significant and notable contribution to element classification in 1869. Mendeleev’s Periodic Law states that the chemical and physical properties of elements are the periodic functions of the elements’ atomic weight.ĭmitri I. The elements that have similar chemical characteristics are grouped in the same column. Metals are to the left in one row (period), while non-metals are to the right. In their properties, there is a repeated pattern known as the “periodic law,” in which members in the very same column (group) exhibit comparable properties. It is arranged in ascending atomic number order. The periodic table is a list of chemical elements arranged in a table. What are the elements? Let’s find out more in the brief study of the periodic table. The periodic classification of elements became vital as a result. In 1865, a total of 63 new elements were discovered. Approximately 31 chemical elements were found around 1800. These elements are arranged in a tabular form in the periodic table.

Update: Here’s a visualization of the categories above.Have you ever pondered that the oxygen you breathe, the different automobile parts, the ornaments you use, and computer semiconductors are all made up of various elements? The modern periodic chart, which is concerned with the study of the environment around us, has roughly 118 elements. For example, cadmium could not be C because that’s carbon, and it could not be Ca because that’s calcium. Many of these elements would cause a conflict if they had been abbreviated using one of the above rules. The next largest group of elements are abbreviated by their first letter and the next consonant, skipping over a vowel. Xenon could have been X, or dysprosium could have been just D, but that’s not how it was done. For example: actinium, aluminum, americium, and argon. There are several elements that start with the same letter, and no element uses just the first letter. For example, helium uses He because hydrogen already took H.

Many of these elements use the first two letters to avoid a conflict with the first letter. When in doubt, guess the first two letters. The largest group of elements are those abbreviated by the first two letters of their name. The easiest abbreviations to remember are simply the first letters of the element names (in English). I included Tungsten in this section because it also has an abbreviation that is mnemonic in another language, in this case German. The elements that have been known the longest often have abbreviations that are mnemonic in Latin.
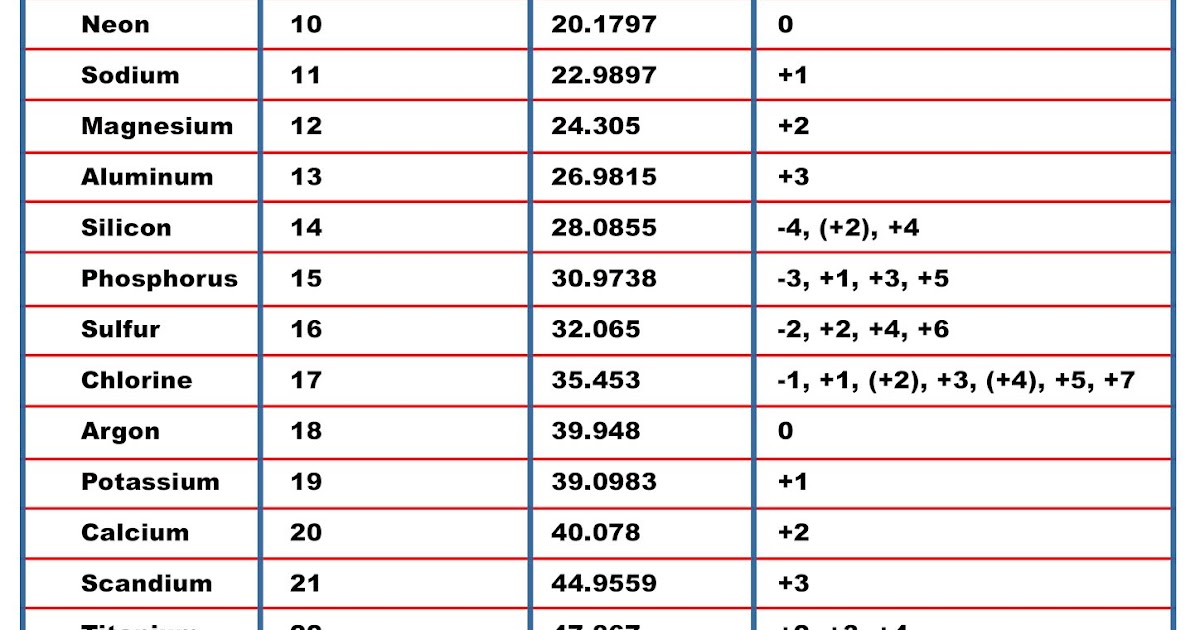
Here’s a survey of how the elements are abbreviated. If you don’t know the abbreviation for an element, is there a simple algorithm that would let you narrow the range of possibilities or improve your odds at guessing? I’ve wondered occasionally about the patterns in how chemical elements are abbreviated.


 0 kommentar(er)
0 kommentar(er)
